Allosteric Regulation of Dynamic Enzymes
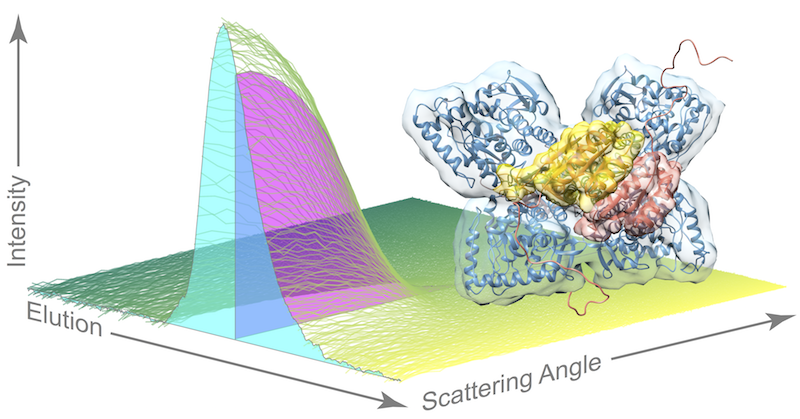
Many enzymes regulate their activity using an allosteric mechanism, where binding of a small molecule causes the enzyme to switch between low and high activity states. X-ray scattering has proven to be a powerful tool for discovering allosteric mechanisms of proteins (Meisburger et al. Chem Rev 2017).
Many important enzymes use allostery to regulate their activity. For example, the liver enzyme phenylalanine hydroxylase (PheH) uses allostery to maintain the proper concentrations of phenylalanine in the blood. Proper regulation of phenylalanine levels is important for human health, and a failure of regulation results in the disease Phenylketonuria (PKU). As part of a collaboration between the Ando lab and Paul Fitzpatrick’s lab at UTHSCSA, I studied how the enzyme changes configuration upon binding its allosteric effector, phenylalanine, and developed a model to explain how regulation occurs (Meisburger et al. JACS 2016).
You can read more about our work on PheH in the excellent blog post written by Tien Nguyen at Princeton: Scientists capture the elusive structure of essential digestive enzyme.