Diffuse X-ray Scattering
How do proteins transmit signals? How do enzymes control multi-step reactions?
The answer these questions, we must find ways to look beyond the average structure, toward the functional collective motions that proteins undergo. To gain new insight into correlated atomic motions, we are developing methods based on X-ray diffuse scattering.
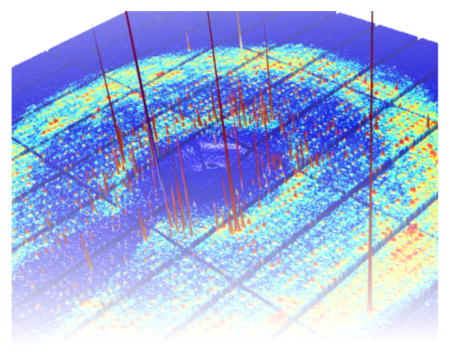
X-ray crystallographers find the average atomic coordinates of a molecule by analyzing the sharp features in the diffraction pattern known as Bragg peaks. However, Bragg peaks are not the only features present in diffraction patterns. When collective motions occur, the patterns also contain diffuse scattering between and underneath the Bragg peaks. Although diffuse scattering is common, crystallographers usually ignore this part of the signal.
Can we make use of diffuse scattering to characterize protein motion?
With state-of-the-art detectors and X-ray sources, it is now possible to map the three-dimensional distribution of scattering from a protein crystal in detail. Our research addresses the remaining “interpretation problem”. We combine precise measurements with detailed simulations to better understand the microscopic origins of diffuse scattering. In addition, we are building new theoretical and computational tools to extract information of biochemical interest.